Novel platform for production of universal flu vaccine
Using our innovative proprietary monoglycosyalated HA (HAmg) flu vaccine technology, we are able to produce uniformly monoglycosyalated HA with a single N-linked GlcNAc at each glycosylation site. Vaccine candidates based on such monoglycosyalated HA have demonstrated critical neutralization activity against various disease pathogenesis.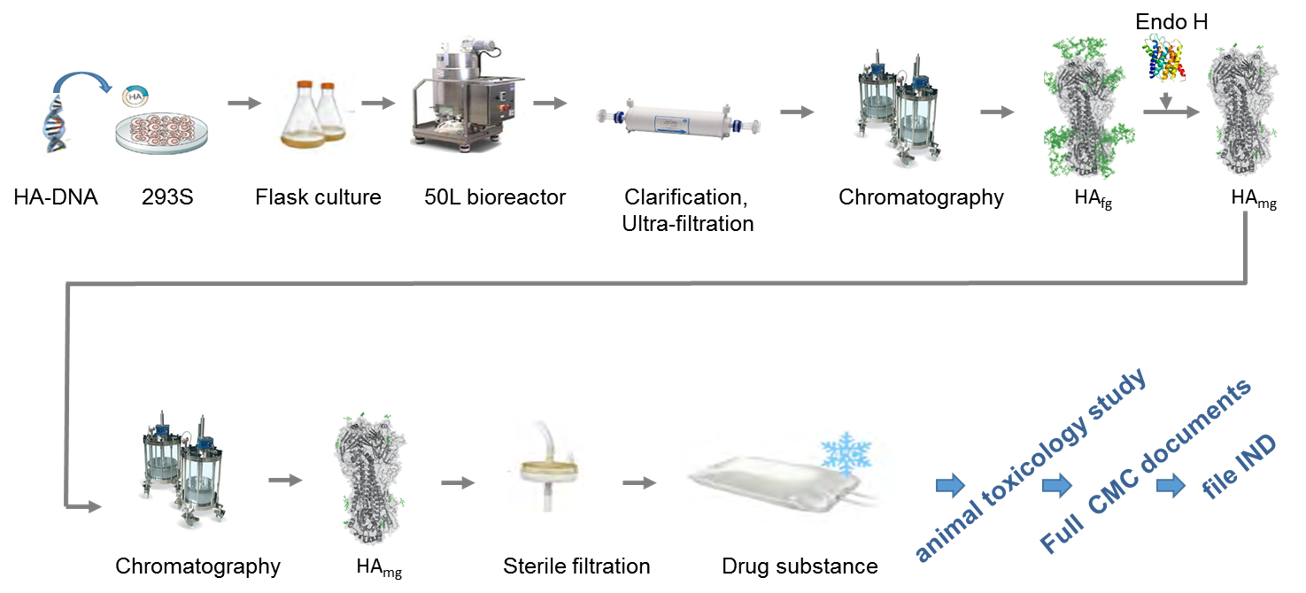
RH Biopharma will supply purified preparation of monoglycosylated HA using established manufacturing protocols suitable for subsequent scale up cGMP manufacture for future clinical trials. The detail process to produce the monoglycosylated HA which is to take the 293S cells for the expression of high-mannose type of HA (HAhm), after HAhm protein purified, the high-mannose glycans were removed from HA by treating with Endo H to be monoglycosyalted HA protein vaccine.
Monoglycosylated HA contributes to better immune responses and superior protection against heterologous strains of influenza viruses
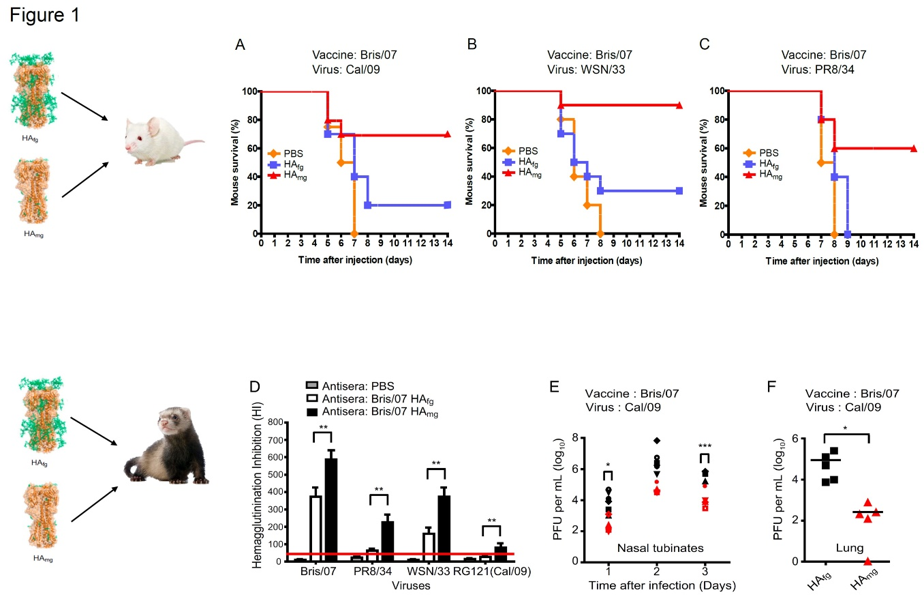
Figure 1 illustrates the impact of vaccination with HAmg offering cross-protection against three H1N1 viruses. Specifically, mice vaccinated with HAmg were challenged with lethal doses (100LD50) of Cal/09, WSN/33, and PR8/34 viruses, and the efficacy of vaccine protection was evaluated over 14 days based on survival rate. After the virus challenge, mice vaccinated with PBS died before day 8 (Fig 1. A-C). A previous study using inactivated Bris/07 virus as a vaccine reported that it provided 30% protection against challenge with the 2009 pandemic A(H1N1) virus. In this study, Bris/07 HAfg showed a comparable level of protection (20%) against a lethal Cal/09 challenge. However, to our surprise, immunization with Bris/07 HAmg offered 70% protection against Cal/09 challenge (Fig. 1A), 90% protection against WSN/33 challenge (Fig. 1B) 60% protection against PR8/34 challenge (Fig. 1C). The cross-strain neutralization and protection induced by HAmg vaccination were also tested in ferrets. The HAmg conferred higher HI titers than HAfg (Fig. 1D). Significantly lower virus titers in the washes of nasal turbinates at days 1 and 3 after infection were observed from the HAmg vaccinated ferrets (Fig. 1E), and virus growth and replication were consistently considerably lower in the lung tissues from the HAmg vaccinated ferrets (Fig. 1F).
Glycans on influenza hemagglutinin affect receptor binding and immune response.
Wang CC, Chen JR, Tseng YC, Hsu CH, Hung YF, Chen SW, Chen CM, Khoo KH, Cheng TJ, Cheng YS, Jan JT, Wu CY, Ma C, Wong CH.
Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18137-42.
Vaccine design of hemagglutinin glycoprotein against influenza.
Chen JR, Ma C, Wong CH.
Trends Biotechnol. 2011 Sep;29(9):426-34.
Vaccination of monoglycosylated hemagglutinin induces cross-strain protection against influenza virus infections.
Chen JR, Yu YH, Tseng YC, Chiang WL, Chiang MF, Ko YA, Chiu YK, Ma HH, Wu CY, Jan JT, Lin KI, Ma C, Wong CH.
Proc Natl Acad Sci U S A. 2014 Feb 18;111(7):2476-81

